-
Posts
113 -
Joined
-
Last visited
Content Type
Profiles
Forums
Developer Articles
KSP2 Release Notes
Bug Reports
Posts posted by JebKeb
-
-
What are your space kraken encounter stories? I recently was 80,000km above kerbin, slowed my orbit to 0m/s and the navball went weird. Not sure if that's a kraken, but still odd.
-
I'm back for a short stint to clear stuff up. Hi.
We seem to have been told that the escape velocity of a black hole at a certain altitude reaches the speed of light. But if the escape velocity is the speed of light, what stops us from being in orbit around a black hole, dipping our periapsis through the event horizon and recording data, then after exiting the event horizon at a higher altitude escape the black hole where the escape velocity is manageable?
Or is the orbital velocity at that altitude faster than the speed of light? Has it been an issue of semantics?
If things get heavier the faster we go could we plunge towards a black hole, reach the same mass as one near the speed of light, and become a black hole? Or would we sling the black hole away at high speed? What would happen?
I'll post more badly formed questions a bit later once I think of some

-
Yeah, that photosynthetic cell looks good. I just wonder about it's efficiency, because natural photosynthetic cells look good. Somewhere in the abstract i read "24% CO faradaid efficiency", but I have no idea what that means. If it is the overall efficiency, then what is more efficient: 2030s solar panels with advanced catalytic reactors, or a 2030s photosynthetic cell?
-
Now before I can start writing anything, I probably have to address that I'm not certain of the price components of fuels. So I won't write anything on the subject I'll get to later.
First clarification of the fuel price components is needed. I'm not sure if these are all the components, but they seem to be to me:
- Fuel tax (And GST? Not sure about in other countries)
- Paying investors for the money used to make the refineries and wells (materials, land, permits)
- Admin costs to keep the people at the top running the company happily and nicely
- Operational costs to pay for maintenance and downtime
- Transport costs
- Station running costs to stay stocked with unhealthy food
- General margin to make investors even happier
- COST OF OIL
Is this about all, or is there far more?
-
I don't know what I'm talking about, I post gibberish. Other people don't understand it, I don't understand their responses.
This has come together in a big mess. I don't even know what to write.
I'm practically certain I'm not describing this clearly enough. It seems that it makes sense to me, but when it goes into text even I don't really understand it.
I think I should spend some time away from this issue and let myself mull over these responses. I'll come back in a while with something far clearer, so I can actually have a conversation.
The chemistry has been sorted out. I now know everything I think I need to know on the processes. In the meantime, a diagram will be sorted out. Then hopefully I can communicate far more clearly in another thread.
-
17 hours ago, Tex_NL said:
Humidifying the dry air only to dehumidify it immediately after makes no engineering sense. Better to just electrolyse the water you already have.
If I didn't do that, then the air processing system would clog up, as I said before.
On 29/07/2016 at 9:00 PM, shynung said:@JebKeb That would mean you're taking energy from the garbage, not the air. Essentially, you're taking a source of carbon (garbage), a source of water, and processing them into syngas, then into liquid hydrocarbons, which is then run through a standard hydrocarbon refinery processes to get fuels and chemicals usually produced by the petrochemical industry.
Waste-to-Fuel system, if you will. The same tech as coal liquefaction, just with different feedstock (garbage rather than coal).
Other than gasification, CO2 can be captured using scrubbers, which essentially absorbs CO2 that passed through it. This CO2 can later be extracted by running hot air through the scrubber.
Also, if you insist to get the the CO2 from the air (to save the environment, say), it's generally a good idea to tap them at the sources: the exhaust flue gas from coal power stations, industrial furnaces, and the like.
The energy was never coming from the air. Amine scrubbing looks like another good bet.
15 hours ago, StahnAileron said:Like others here, I see no practical purpose to the humidification/dehumidification cycle. Your point it making the dew point higher just means you'll make it "easier" to extract the water from the air because you added more water. Dew point scales with the concentration of vapor in the air, so you'll still have the same dew point after you remove the moisture you added. This is an extraneous process that won't do anything other than waste energy. Just dehumidify the air. Or better yet, just get a ready source of liquid H2O. Same with the CO2 source, really. Unless your goal is to also have some form of CO2 capture built into the process for whatever reason.
Oh, BTW, liquid CO2 is impractical to produce. It takes over 5 atmospheres of pressure at around -60 degrees Celsius to produce CO2 in liquid forms. Easy enough to actually do (5 atmospheres is around 75psi; you can put that much pressure in a bicycle tire), but it's also probably easier to just freeze the CO2 directly out of the air at around the same temperature, move it, then reheat it to gas form.
As mentioned, electrolysis is a power-hungry process. You're not gonna get much back on your power investment. Although:
...isn't quite true. Generate enough voltage and anything will eventually become a conductor. (If horribly inefficient. Or you wind up with a plasma...)The only thing I can think of that is a "perfect" (electrical) insulator is a perfect vacuum. And that's only because you don't have electrons (and therefore charges) there in the first place to kick around. (Huh... So the literal "nothing" is the perfect insulator, now that I think about it...)
Something I probably want to mention: I am NOT taking all of my water from the air.
Anyway. Is this maths faulty?
80,000bpd = 150l/s
150l x 45MJ/l = 6750MJ/s
1kj = 1kw second
1kw second = 1kw
so 6750MJ = 6750MW.
Let's assume solar panels are $0.50/w.
Total cost of system: $3.4 billion. Cha-ching
-
21 hours ago, shynung said:
If the air is dry, it's much more efficient to look for places where the air is more humid, rather than injecting water into the intake air.
Also, what are the inputs and outputs? Only ambient atmosphere, or can it use other resources available nearby? What are the expected products?
Seeing how bad the energy scenario is playing out, I think I'll also be burning garbage to produce heat and carbon dioxide for the Air-To-Fuel system. (That's what I'm calling it.) It'll take in salt/seawater and water as well.
-
With a bit more stuffing around the cost appears to have dropped from $200 billion to only $200 million. It still requires a 3.5 kilometre field, but it's a LOT smaller than the original, which was 13.5km! We can probably make it even smaller with wind and tide.
-
I dehumidify the air so it doesn't clog up the air processing.
15 hours ago, YNM said:Fractionate an azeotropic solution ? Really ?
Only with the propanol fraction.
I've stumbled across something extremely peculiar. Take the following Fischer-Tropsch reaction:
2 CO + 5 H2 -> C2H6 + 2 H2O
CO takes 38kj/mol to produce, and high temp electrolysis produces H2 for 216kj/mole. This means, per unit of feedstock, it takes 1156kj/unit to produce. What I've noticed is ethane produces 1560kj/mole in combustion, so this seems to leave a surplus of 404kj.
404? That does seem suspicious...
-
14 hours ago, YNM said:
You can't fractionate alcohols out of water. Also, why would you humidify then undo it again...
Fractionate alcohols out of the water-alcohol solution, I should have wrote. Also, the dew point is too low sometimes, so the water would freeze. Putting some water in makes the dew point higher.
14 hours ago, wumpus said:Is this meant to be on Mars? You simply skip most of that humidification/dehumidification process by merely choosing a non-desert climate on Earth and tapping a river filled by rain (a natural process that does all that for you).
Also the subject is rather misleading. "Fuels" often imply an energy source, what you describe appears to be an energy sink (or more accurately, a low efficiency form of energy storage). Obviously for lifting off Mars (or similar), it might not matter how inefficient your process is, as long as the end concentrates enough power to have TWR>>1
And as far as "keeping it to engineering", I'm glad I wasn't the one responsible for the debacle that caused my Chief Engineer's livid rant about "price is a spec"*. If you can't get the price of this fancy system under the price of an oil well, it won't be a success. There will be oil wells still around once there exists enough power from other sources to power this expensive process. The only hope for this process is that the desire for fuel (for aerospace mainly?) and chemical feedstocks (and plastics if they use different wording) require more petro-products than the remaining oil wells can supply.
* You better believe heads rolled. And more than a few laid off who had nothing to do with it, merely because of the lack of money it caused.
In no way on mars!
I'm presently reviewing the entire energy situation on this idea.
-
Now, if there are any comments about the economic viability of this, please go back and post them on the original thread. I want this to be a chemistry and engineering only discussion.
I've been developing a system to produce synthetic petroleum for quite a while. I first showed it to the KSP community on the original thread. Now, I'd like to iron out the chemistry until it's all sorted and I can write it up completely. (This is no university project, just a hobby.) I'll write out the concept and put italics on things which I am not certain of.
This is now the concept. Air is taken, and if it is dry, water is injected to provide a dew point above the freezing point of water. The air is heat exchanged with a coolant, then it travels along a large, inclined set of pipes with holes to extract humidity. The humidity is purified to near pure H2O, then mixed with all the other streams of pure water from around the plant. It then is electrolysed into hydrogen and oxygen for use.
The dehumidifies air is now compressed until the carbon dioxide is a liquid. It then goes down a similar system to the dehumidifier. The now pure air is vented. This carbon dioxide is taken, mixed with hydrogen and fed to a Sabatier process reactor, which produces water (which is drawn off) and methane. Some of the methane is odourised and pumped into the natural gas grid. Most of it is taken and mixed with pure oxygen, then catalytically partially oxidised in a reactor. The product of this reactor is syngas. The syngas is now altered to have desirable constituency, then it is fed into a Low Temperature Fischer-Tropsch reactor.
The LTFT syncrude is split in a reflux drum into gas, syncrude and water. The water has a large amount of alcohols in it, and these are seperated via fractionation into very pure chemicals. The syncrude is hydrotreated to turn alcohols into paraffins and water. The hydrotreated syncrude is then mixed with the gas, then put into a huge distillation column. Light naptha is isomerised, heavy naptha is catalytically reformed. The kerosene, diesel and lube fractions' fate has not yet been decided.
The gas rising out of the column is pressurised and fractionated. Unused syngas goes back to the LTFTR. CO2 goes to the Sabatier reactor. Methane is CPOX'd. Ethane is cracked into ethylene, then blended in with the ethylene. Propane and propylene go on their merry ways. Butene and butane do something else. The butane is isomerised with the light naptha, then the isobutane and butene are alkylated into a high-octane petrol blending stock.
Atmo residue is taken to a vacuum distillation system. The light wax is put through a FCC, producing high octane crackate and cycle oil, which is mixed with the heavy wax and hydrocracked. The vacuum residue has an unknown path. Concept ends.
Now I shall submit my questions.
- Are there any other methods of producing syngas, not including gasification?
- Are there any non-sulphur using odourants for LPG and natural gas?
- What would be the most efficient method of capturing carbon dioxide and water?
- Would it be possible to use hydrogen to cleave C-O bonds in alcohols?
- What other things would be required to refine the kerosene, diesel and lube fractions?
- What is the composition of FCC product?
-
What are they? And how do I get them?
-
I appear to have forgotten a LOT.
I've got several VERY important things to know. Firstly, what is the product composition in FCC product?
Next, how would I remove longer alcohols from the syncrude? I have 2 methods.
- Add solvent to the syncrude. Seperate the solvent, water and light alcohols in a reflux drum. Deoxygenated syncrude heads onto the distillation column.
- Seperate water and light alcohols in a reflux drum. Send through a hydrotreater which splits the C-O bonds in alcohols using hydrogen, and also hydrogenates olefins into paraffins. (Olefins lead to aromatic formation in FCC and catalytic reforming, my spies tell me, so more paraffins would be desirable.)
Fjnally, what other oxygenates are found in LTFT syncrude? Sasol appears to have ketones as well as alcohols in their diagrams.
-
Well, it looks like everything has been cleared up and chemistry has been sorted out. I'll be starting a new thread soon, with a summary of the entire concept. That will probably be the thread where everything is ironed out and economics are explored. I'll link to it in the next post.
-
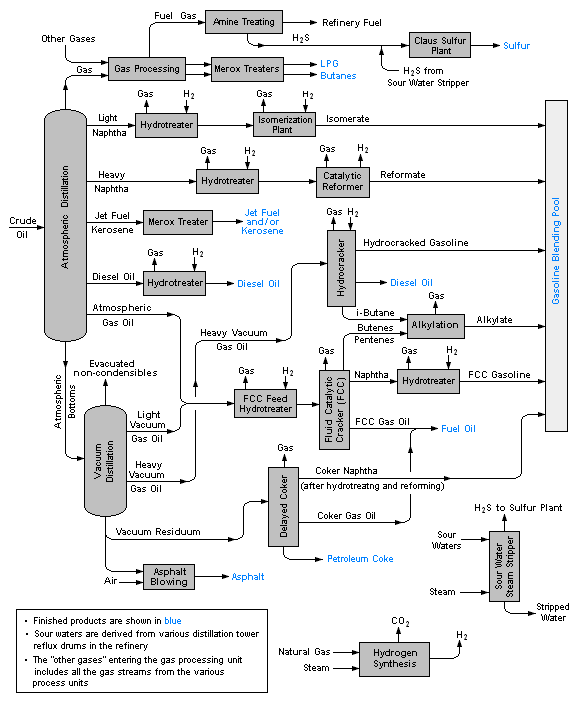
What does this gas processing at the top involve and where can I find a diagram for it?
-
Probably not, unless it is oxygenated into ethanol. Also, it's a gas, so transport is a bit complex.
-
What about as a kind of visbreaker, could we use a hydrocracker to isomerise and crack the heavy hydrocarbons in LTFT syncrude? Also, would that hydrogen force it's way inbeween the e.g. CH3 and OH to give us methane and water from methanol?
-
Let's just shift back to the low-temp FT process.
It appears to produce around 55% water, 40% hydrocarbons and 5% oxygenates. These hydrocarbons are around half/half paraffins and olefins. I was thinking, could you use a hydrotreating unit to turn olefins and alcohols into paraffins? I'm pretty certain we could easily hydrogenate the olefins, but adding H2 to alcohols I am wondering about.
CH3CH2OH + ? H2 -> 1,2
1 - CH3CH2OH3 -> CH3CH3 + H2O
2 - CH3 + CH4 + OH -> CH3OH + CH4
CH3OH + H2 -> CH4 + H2O
Is this in anyway possible?
-
I've been told this many times and I am wondering whether it is true. Here's my analysis.
Money in the US is made from about 75% cotton and 25% linen. Cotton is practically a special form of celluose, and linen is pretty similar. The combustion energy of celluose is 2828kJ/mol, and one mole of celluose is 630g, so we'll estimate and say 4mJ/kg due to the impurities in cotton and linen.
Celluose probably has a pretty low isp, so I'm estimating it at 100 due to lack of data. To get 1 tonne to orbit, assuming a very light container, we'll need about 50 THOUDSAND TONNES of fuel! Let's say 60K, because of the complex machinery to combust such a non-rocket-fuelly propellant.
That's 50 tonnes of cash per kg to space. Let's assume we're using 100 dollar bills, weighing 1g each, requiring $5,000,000,000 per kg to space. Present launch costs are on average about $12K/kg.
Here is a list of different costs to orbit.
- 1 dollar: $50,000,000/kg
- 2 dollar: $100M/kg
- 5 dollar: $250M/kg
- The rest are obvious, so...
- 1 trillion dollar: $50,000,000,000,000,000,000/kg, or the GDP of 500,000 Earths.
So, it looks like this example is far off. Let's see if there are any other comparisons.
-
On 05/07/2016 at 7:20 PM, Cirocco said:
When you say Co, do you mean carbon monoxide or a Cobalt catalyst? Or something else entirely?
Cobalt catalyst. I am using the formula of things.
-
I've been looking at another method of producing synthetic fuels involving the dehydration of methanol, the Mobil Process.
First, methanol is dehydrated over SiO2 and Al2O3 to yield a mix of methanol and dimethyl ether (isomer of ethanol). Then it's dehydrated completely over ZSM-5 into ethylene and methylene, and oligomerised into a mix of branched paraffins and olefins, napthenes and aromatics. I think the catalyst structure determines the maximum length, so using different zeolite catalysts you could produce kerosene or diesel, as well as petrol.
Then I noticed that the Fischer-Tropsch process is practically decomposing CO into C and O and hydrogenating these into water and methylene, which then oligomerises into straight-chain paraffins and olefins.
So, could you make an "advanced FT process" which uses the first few steps of FT through Co, then oligomerises the products over a zeolite catalyst providing much easier refining? Also, by blending the mixes of Co and different zeolites could you change the composition of the syncrude?
-
On 5 June 2016 at 8:21 PM, RandomUser said:
Hah, that'd be pretty funny. The sad thing being that they'd still continue to say that it is all Photoshop, or they'd just reiterate their nonsensical theory to fit more with what they'd seen. Flat Earth believers aren't, and have never been, of any immediate or future threat to knowledge, or people, otherwise somebody would go through all the effort to prove them wrong. But it's just the simple fact that is not a true issue, therefore nobody or conglomerate of people (NASA, ESA, etc.) are willing to go through all the effort.
Though, one really has to wonder how such an absurd theory as this could be entertained by even the most little amount of people, let alone enough to gain media attention.
Stick 'em on the outside with no helmet so there's no distortion.

-
Thanks for asserting he's not to be trusted. That bit on cognitive dissonance at the side of the homepage was a bit...savage?
He thinks the world is round and believes in planets, but doesn't think they move and only spacecraft are affected by gravity.
-
On 22/06/2016 at 8:42 PM, Cirocco said:
Well there's your problem. Waxes are reeeeeaaaaaally hard to crack properly into usable fractions. Heavy hydrocarbon chains, sure, you can crack off a couple carbon atoms from the edges and turn them into lighter fractions and gas (which is usually just burned with a large torch on top of the refinery). But with waxes, you need to selectively crack the heaviest ones down the middle without breaking up the rest of the chain and without cracking the lighter ones. And that is exactly what is so hard: cracking long chains without cracking the small ones into useless, short-chain gasses. Add to that the energy requirements being so restrictive, and there's your explanation for why this process isn't widely used. It's simply still cheaper to just drill it up.
Thanks for clarifying what cracking actually is - it's cracking molecules into fractions, not set sizes.
Vacuum distillation would probably be good to seperate out the wax into different grades.
Just being curious, do different conditions during cracking give you different gases?



Galileio Burnup on Jupiter
in Science & Spaceflight
Posted
I found a rather interesting page that said something along the lines of the plutonium pellets fell through jupiter, reached crush depth and ignited, causing the splotch that appeared 1 month after the burnup of Galileio.
Now this guy seems a bit conspiracy-theorist in other articles on his website, but this page, apart from the end, seems balanced and mostly true.
Anyone got an opinion on this?